Our specialist team develops collagen in a wide range of formulations to suit any application or research goal.

Liquid Collagen
Collagen Type 0 in liquid form at a range of concentrations dependent on the user’s needs (illustration shows concentrations from 5 to 15 mg/mL) and can be produced and supplied for applications in areas such as bioprinting and tissue engineering.
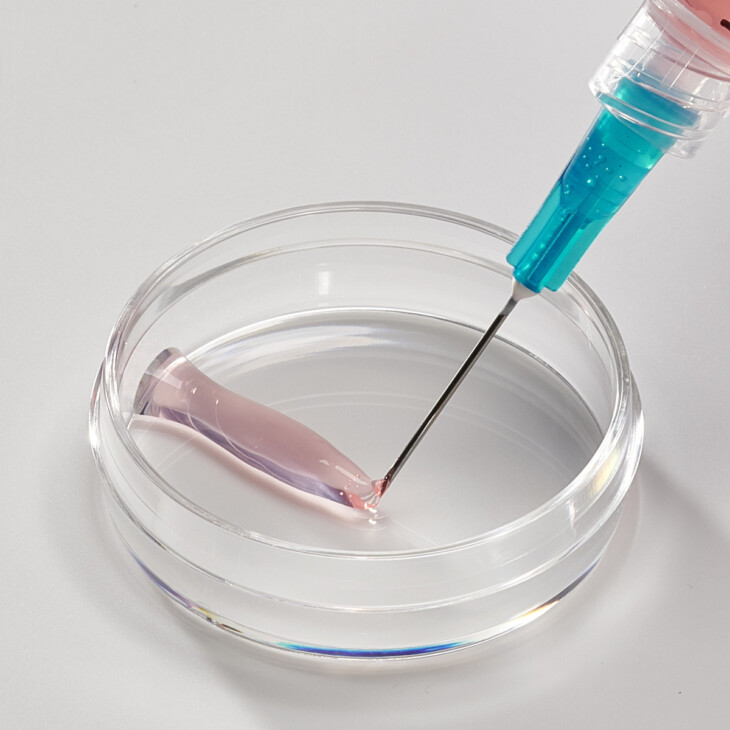
Hydrogels
Neutral, isotonic solutions of Collagen Type 0 results in the formation of viscous liquids that can be formulated into viscoelastic 3D hydrogels that can be administered through various routes, including as an injectable (utilizing crosslinking technology).
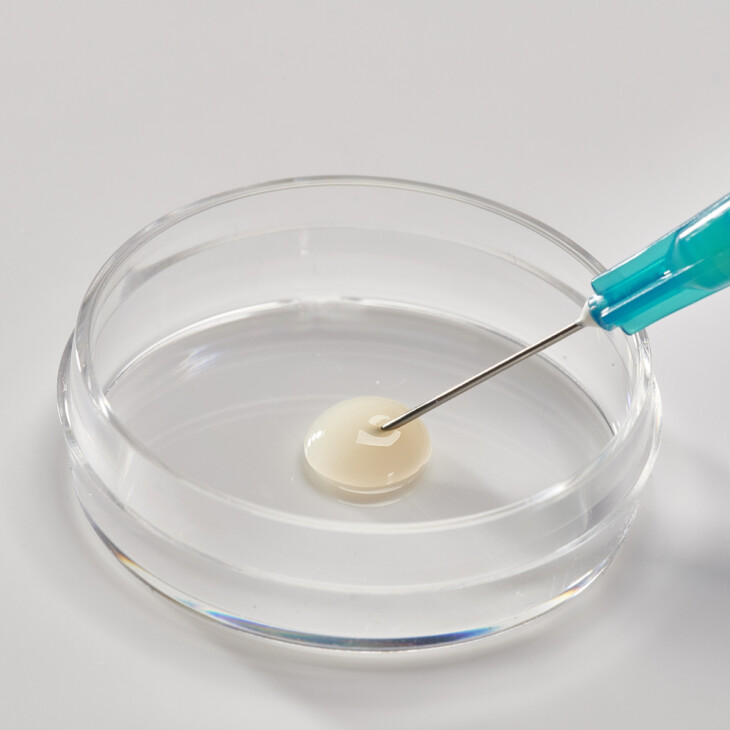
Flowable Matrix
Collagen powder can be formulated into an injectable device / flowable matrix through suspension in solution according to customer requirements. Flowable collagen matrix can be used as a wound or dermal filler.
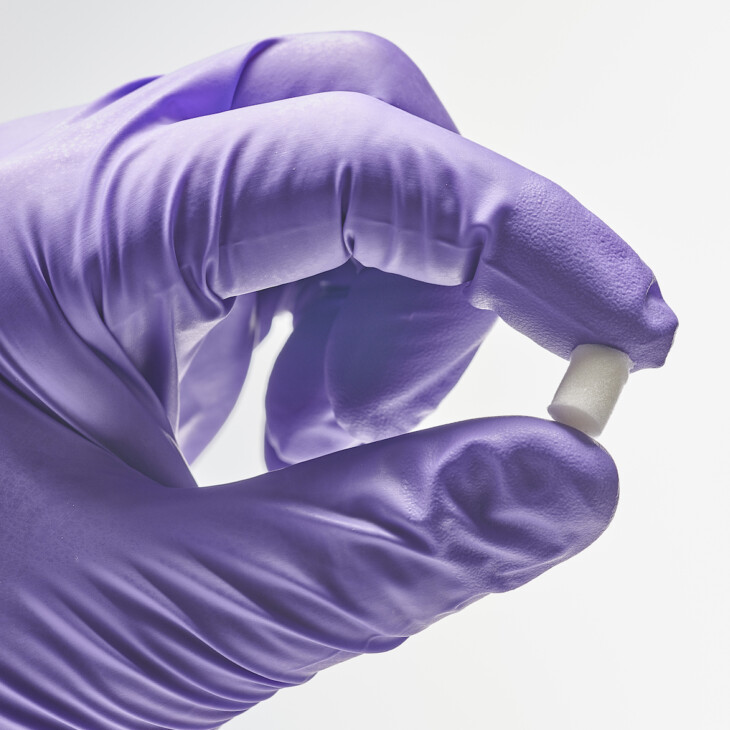
Scaffolds
Lyophilised scaffolds comprising Collagen Type 0 can be utilized for various applications and reconstituted using various liquids to achieve a robust hydrogel matrix. Crosslinking can be applied to the scaffolds and their size/shape can be controlled to generate custom dressings etc.
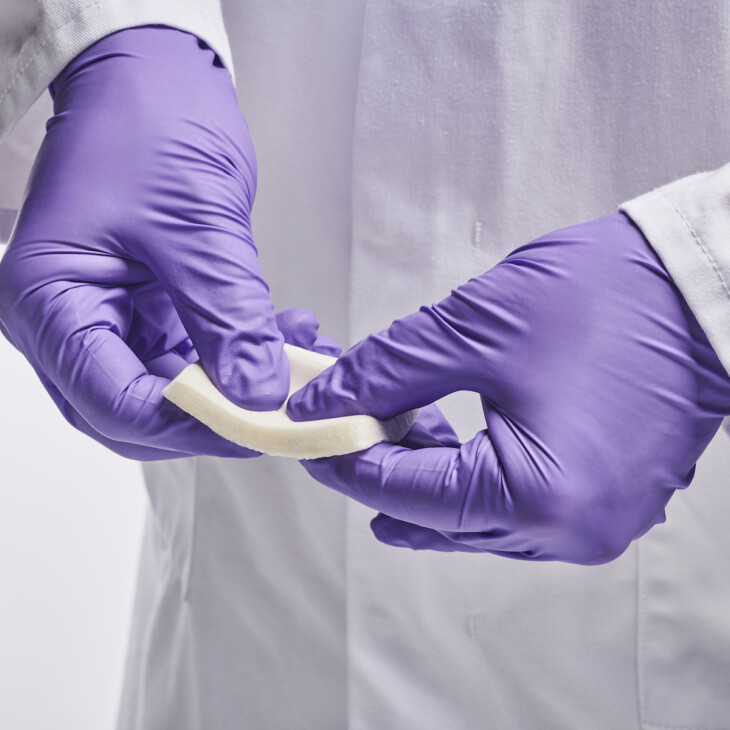
Dressings
Wound dressings and patches can be prepared from Collagen Scaffolds (including composite scaffolds).
Get in touch
If you would like to know more about Collagen Type 0, or have any other questions, please contact us.

Collagen Type 0 Whitepaper
Find out how Collagen Type 0 can revolutionise Regenerative Medicine
In this whitepaper we explore:
- Where Collagen Type 0 comes from
- Properties of Collagen Type 0
- Biocompatibility and anti-inflammatory properties of Collagen Type 0
- In Vivo wound case study
- Clinical Applications for Collagen Type 0